Frontline healthcare workers administer critical medical oxygen to their patients every day. But where does the oxygen come from?
This question is more pertinent than ever with recent challenges like the COVID-19 pandemic and natural disasters affecting oxygen supply networks. Understanding the basics of the medical oxygen supply chain equips facility managers, respiratory therapists and other healthcare personnel with the knowledge they need to mitigate the risk of a future supply disruption.
In this three-part series, we will follow bulk medical oxygen from the plant to the patient.
Click below to jump to the most relevant section:
Part 1 - Medical Oxygen Production and Delivery
Part 2 - Components of the Bulk Medical Oxygen System
Part 3 - Three Steps to Identify Medical Oxygen Flow Restrictions Inside Your Healthcare Facility
Part 1: Three Questions Every Healthcare Facility Should Ask About Bulk Medical Oxygen Production and Delivery
When evaluating your medical oxygen supplier’s reliability of supply, here are some questions to consider:
- How close is my primary medical oxygen source to my hospital and are there backup sources nearby?
- What is my medical oxygen supplier’s emergency preparedness plan for customers who experience equipment failure or oxygen surges?
- Does my supplier provide remote monitoring of my bulk system to optimize deliveries and can I easily get access to that data?
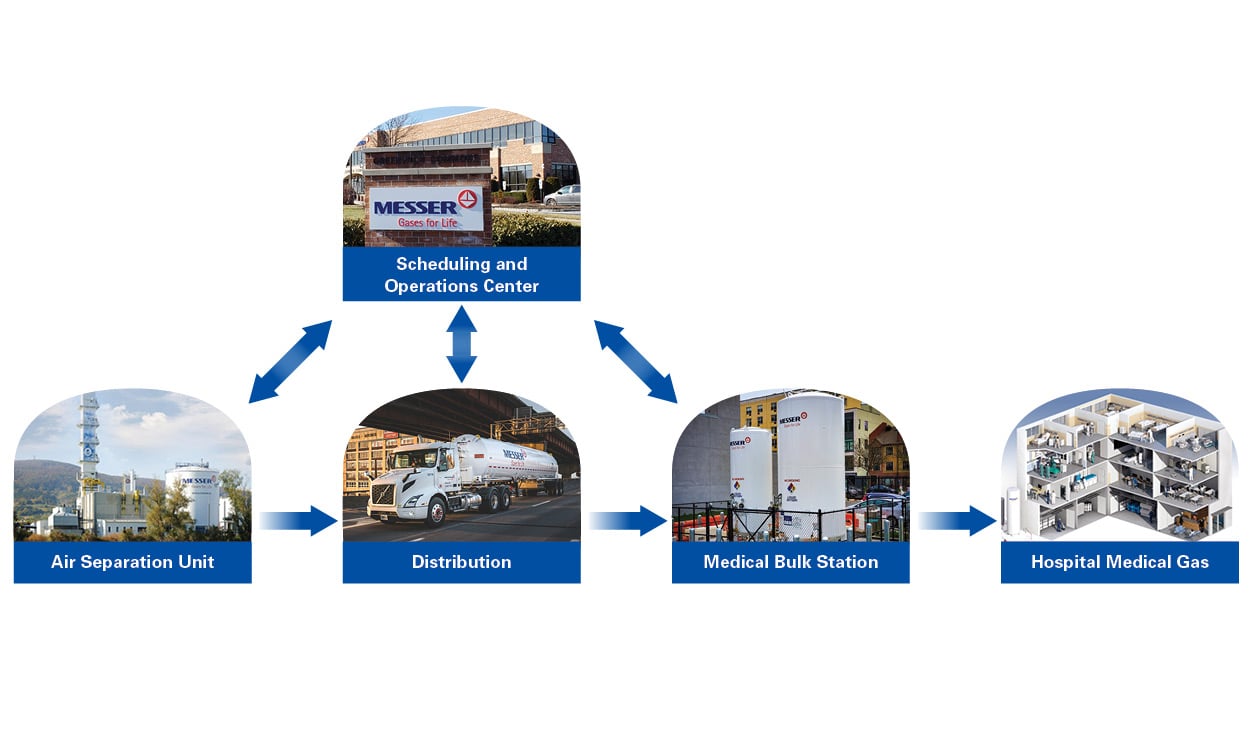
Medical Oxygen Production and Distribution
Prior to medical oxygen arriving at a healthcare facility, medical oxygen suppliers take crucial steps in producing, analyzing, and distributing oxygen in compliance with regulatory and industry standards. Medical oxygen suppliers produce oxygen bycompressing and separating air into nitrogen, oxygen and argon through a cryogenic distillation and purification process.
Messer utilizes its vast supply network of plants and sources to produce medical oxygen throughout the United States. With numerous plants and sources located in contiguous geographies, Messer supports its customers using backup supply sources. Because bulk medical oxygen is classified as a drug under Title 21 of the U.S. Code of Federal Regulations, all medical gas manufacturers are required to register each of their air separation plants annually with the Food and Drug Administration (FDA).
The Compressed Gas Association (CGA) and United States Pharmacopoeia (USP) specify the manufacturing and analysis of USP (medical) oxygen to ensure impurities are removed to a required level and the oxygen is suitable for patient care. It is the responsibility of the medical gas manufacturer to ensure its standard operating procedures (SOP) comply with all federal, state, and local regulations. Bulk medical oxygen storage tanks are sampled daily to verify conformance to specification. This specification requires that the oxygen purity exceed 99.5% purity and is free from foreign odor. Once the medical oxygen is loaded into the trailer, it is then analyzed again to confirm that it meets or exceeds the specification and a Certificate of Analysis (COA) is provided to the hospital with each delivery. The COA should be stored in hospital records for use and reference in future inspections, such as those performed by Joint Commission, a healthcare accreditation organization.
For more information about manufacturing medical oxygen, please refer to the following resources:
- Current Good Manufacturing Processes Practice for Medical Gases
- Standard For The Manufacturer Of Bulk Medical Gases - 4th Edition (CGA M-3 2015)
- FDA Compliance Program Guidance Manual on Compressed Medical Gases
Medical Oxygen Delivery Scheduling
As the COVID-19 pandemic swept across different regions of the U.S., Messer monitored and responded to unprecedented demands in medical oxygen usage. Messer quickly mobilized to increase production of oxygen, re-allocated drivers and bulk delivery trailers to areas where they were most needed, and increased delivery frequency to its healthcare customers to keep pace with the surging demand for oxygen. Messer also deployed portable oxygen trailers to provide supplementary supply to hospitals experiencing increased demand.
Messer provides an optimized supply chain for a healthcare facility’s medical oxygen needs. During the pandemic, hospitals learned that it can be advantageous to have separate bulk and cylinder suppliers to diversify their sources of supply. Because the hospital received oxygen from two different oxygen supply networks, both oxygen suppliers could flex their networks to supply bulk oxygen.
Messer’s Operating and Scheduling Center in Stewartsville, NJ controls and monitors plant operations remotely to consistently produce and deliver medical oxygen for our medical customers. The Operating and Scheduling Center operates 24/7, 365 days per year, and has a Disaster Recovery and Emergency Response Plan that features redundant telecommunications, data connectivity and an additional diesel generator for electrical back-up to ensure uninterrupted service and support for our customers.
Messer monitors medical oxygen usage patterns remotely via telemetry systems to help optimize delivery schedules. Our customers can view their telemetry data and delivery history on our proprietary NSC Online platform. Please reach out to Messer to learn how to access your oxygen usage information.
What Makes a Successful Medical Oxygen Supplier?
Premiere medical oxygen suppliers must have reliability of supply, strict SOPs to ensure patient safety, a strong emergency response plan and a remote monitoring system. A superior supplier presents the lowest risk option while not compromising the quality of the product or the customer’s unique needs.
Continue reading for part two in our three-part series to learn more about bulk medical storage equipment design and piping systems to help you identify potential bottlenecks in your current oxygen delivery system.
We deliver to you, so you can deliver to your patients.
Part 2: Understanding the Major Components of Your Bulk Medical Oxygen System
Once your medical bulk oxygen supplier has delivered oxygen to the healthcare facility, how do healthcare facilities get oxygen to their patients?
Given medical oxygen is critical for life-support, bulk medical oxygen systems are designed and installed with redundancies, alarms and monitoring to respond to maintenance issues and maintain consistent supply. The systems should be installed and operated in accordance with the code specifications of National Fire Protection Association (NFPA) 99, the Health Care Facilities Code, and NFPA 55, Compressed Gases and Cryogenic Fluids Field Code.
What Are the Components of a Bulk Medical Oxygen System?
A typical Messer medical bulk oxygen station at a hospital features a main and reserve bulk oxygen tank, main and reserve vaporizers, a regulating manifold, and a local alarm panel installed on a specified foundation.
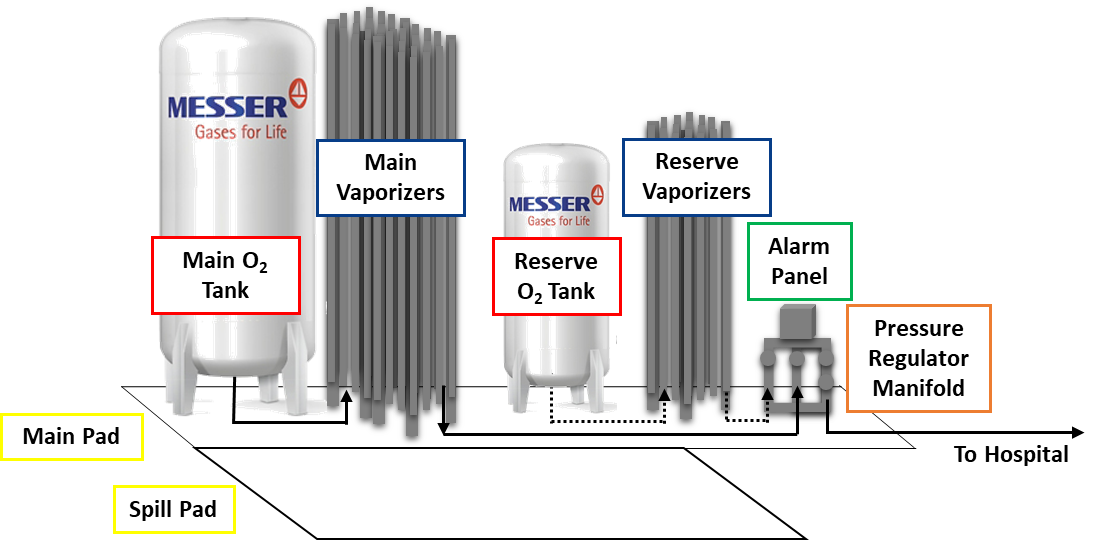
1. The Main Tank
Given the Covid-19 pandemic, more care needs to be taken with regards to choosing the size of the oxygen tank. High flow oxygen treatments rates along with clarification of the number of possible simultaneous treatments sets the daily maximum. While NFPA 99 does not specify main tank sizing, many suppliers suggest the tank be sized with at least a two-week supply of medical oxygen. This allows the healthcare facilities to maintain adequate medical oxygen inventory and suppliers to optimize deliveries. The main tank size may increase depending on specific hospital needs and risk tolerances for such emergencies as pandemics and natural disasters.
2. The Reserve Tank
If the supply from the main tank is interrupted for any reason, the reserve tank is designed to automatically engage as a back-up tank.
- NFPA 99 2021 requires that the reserve tank hold at least a 24-hour supply and this should be based on maximum flow rates.
- It’s highly recommended to have a minimum of 48-hour supply, based on a hospital’s oxygen demand. When the demand is unknown, your supplier may be able to make a recommendation on size and quantity.
If a healthcare facility’s oxygen usage is minimal, reserve oxygen cylinders may be used in place of a reserve bulk oxygen tank. Per NFPA, both cylinders and bulk tanks are acceptable reserve supply systems.
3. The Vaporizers
Vaporizers receive liquid oxygen from the bulk oxygen tanks and convert it into gas to feed the healthcare facility’s oxygen pipeline system. Many suppliers suggest the installation of vaporizers with separate circuits for the main and reserve tanks in order to provide redundancy. Vaporizers are usually atmospheric heat exchangers; they use ambient air to convert the oxygen from a liquid to a gas. Steam, water bath and electric vaporizers provide alternative means of vaporization in situations where the healthcare facility might require a custom solution. In these cases, ambient reserve vaporizers should be utilized to maintain continuity of oxygen supply in the event of a power failure or other issue.
Excessive frost can accumulate on vaporizers during surging demand or cold weather conditions. Heavy icing can reduce vaporizer performance; report heavy icing to your medical oxygen supplier so they can help with de-icing the vaporizer. Please refer to the blog entry on identifying heavy icing for more information.
4. Pressure Regulator Manifold
The manifold regulates oxygen pressure for use inside the healthcare facility for patient care. For example, respiratory treatments usually require 50-55 psi oxygen while hyperbaric therapies often require 70-90 psi.
- NFPA 99 2021 (NFPA 99 2021: 5.1.3.5.5.1) requires dual final line regulators on the manifold to allow for redundancy if one regulator needs repair.
- Your supplier can provide a separate manifold for each pressure requirement within your facility.
5. Alarm Panels
NFPA 99 requires an alarm scheme to monitor bulk oxygen system operations and to notify the facility of potential supply issues. In a key provision, NFPA 99 2021: 5.1.9.2.1 requires two master alarm panels inside the hospital (details to follow in Part 3 of this blog series). Messer supplies a local alarm panel on the regulating manifold to provide another location for the facility to review the bulk oxygen alarms.
There are four alarm signals originating from the medical bulk oxygen system:
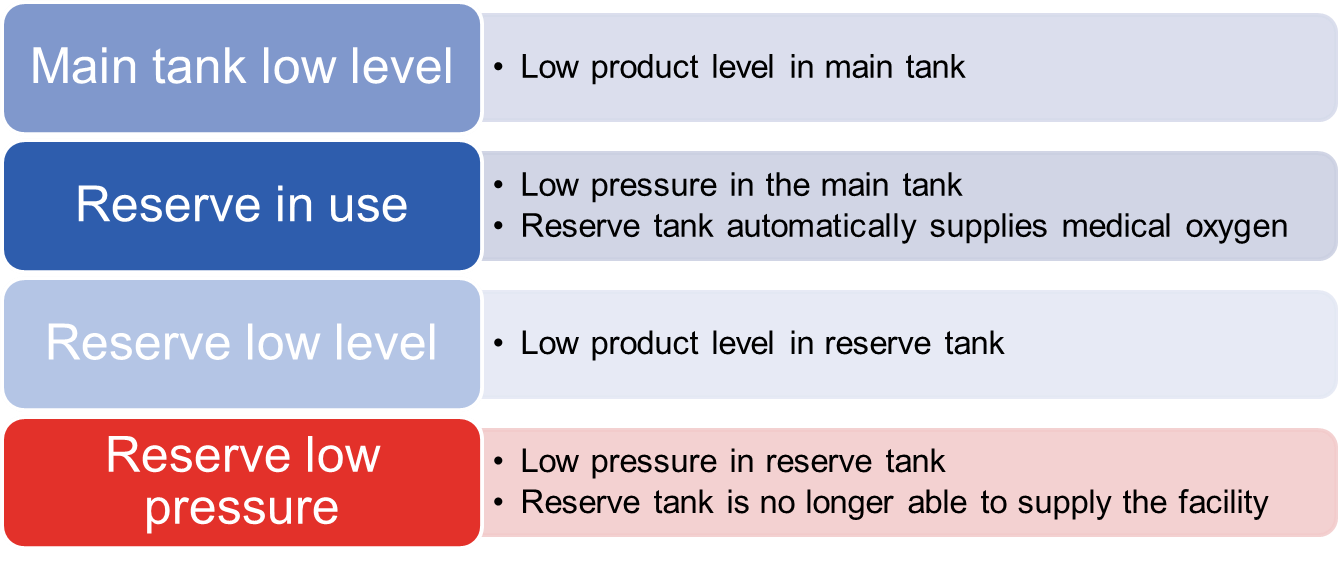
If you receive an alarm, call your bulk oxygen supplier. If Messer is your supplier, call Messer’s emergency line at 1-800-232-4726, open 24/7, 365 days a year.
If Messer is your supplier, we work proactively to maintain a consistent supply. As mentioned in the first part of this medical oxygen blog, we monitor medical oxygen remotely via telemetry systems to help optimize delivery schedules. You can view your telemetry data on our proprietary NSC online platform. Reach out to us; we’ll show you how to access your oxygen usage information.
6. Bulk System Pad
Due to the inherent safety risks with liquid oxygen (check out our oxygen safety video for more information), NFPA 55 requires specific safety distances between bulk oxygen tanks and other safety hazards like combustible materials and powerlines. The code also requires a 12 foot x 12 foot non-combustible surface for oxygen delivery (a spill pad). For the main pad, code specifies 3-foot distances between each piece of equipment, fencing with a second means of egress inside the bulk system enclosure. Work with your industrial gas supplier to confirm that it is accessible and meets code requirements.
What to Look out for When Monitoring Your Bulk Oxygen System:
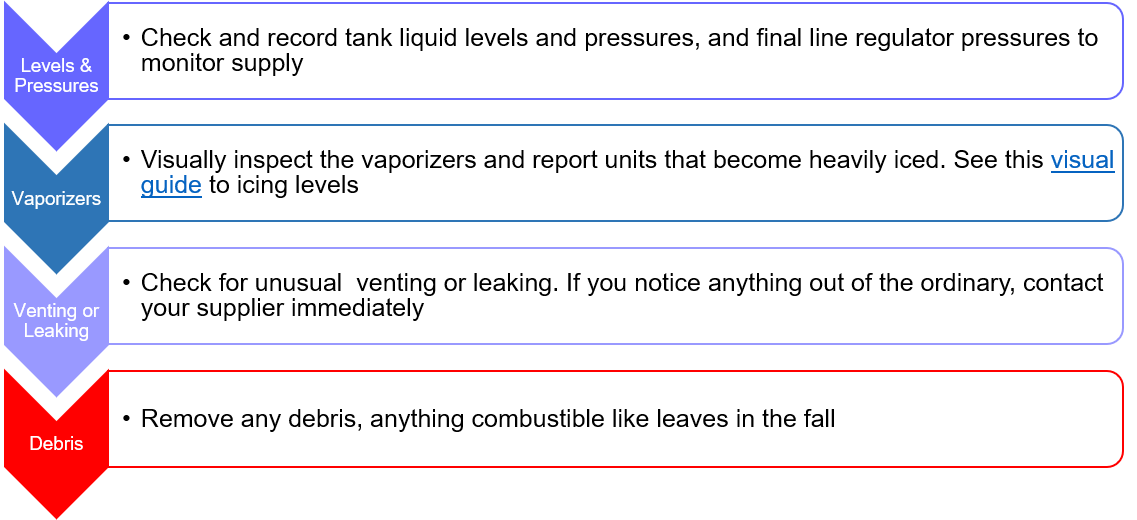
While the alarm systems are designed to maintain continual supply, healthcare facility personnel also have a role to keep everything running smoothly. Regular inspection of your medical bulk oxygen system is key.
Messer recommends these four inspections daily:
Messer recommends these activities annually:
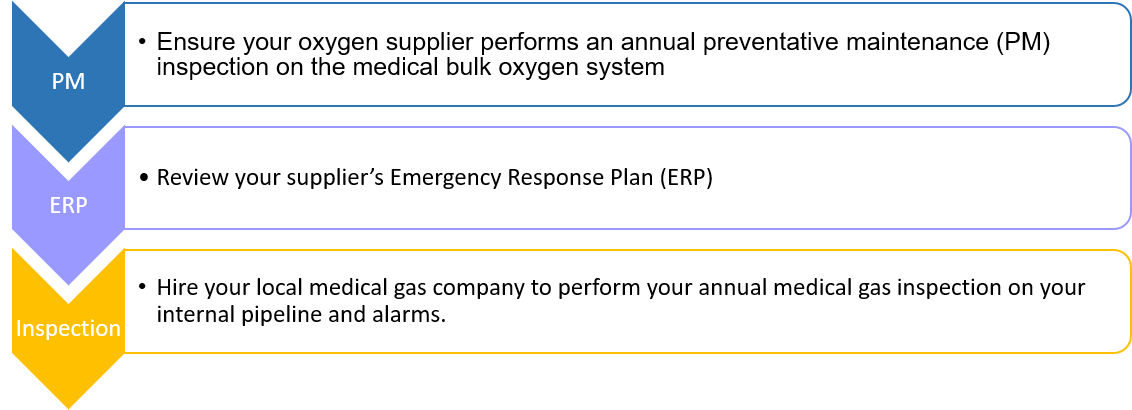
Make sure you understand your medical bulk oxygen system. Ask your supplier for training, and to walk you through the components of the system. Messer provides in-service training at the commissioning of a new medical bulk oxygen system and as part of its annual preventative maintenance services. If you have questions or concerns, contact us for a review of your system.
Part 3: Three Steps to Identify Medical Oxygen Flow Restrictions Inside Your Healthcare Facility
In parts 1 and 2 of Messer’s 3-part series we looked at the production and delivery of medical oxygen to your healthcare facility, highlighted components of a typical medical bulk oxygen system, and discussed the National Fire Protection Association (NFPA) codes that help ensure a safe and reliable supply of medical oxygen. In this final post of our series, we will go inside the hospital to follow the medical oxygen to its destination: the patient.
Once the medical oxygen reaches the source valve, usually located on the bulk oxygen pad foundation, it becomes the healthcare facility’s responsibility to get this critical gas to patients when they need it.
How do hospital engineers and facilities and respiratory personnel keep medical oxygen flowing at the necessary pressures? How do they assess potential restrictions downstream that may impede flow, especially in an unexpected surge situation such as the COVID-19 pandemic?
Drawing on Messer’s specialized experience and know-how as well as the medical gas system design experience of H.F. Lenz Company, Johnstown, Pa., we’ll highlight common oxygen flow restrictions and the relevant National Fire Protection Association (NFPA) 99 2021 Health Care Facilities Code updates to help maintain a continuous and reliable medical oxygen supply.
Take these 3 steps to be sure your medical oxygen is available to your patients when they need it most.
Step 1: Identify the causes of flow restrictions in medical oxygen piping
As detailed inPart 2: Understanding the Major Components of Your Bulk Medical Oxygen System and our “Five Steps for Hospitals to Help Manage Increased Medical Oxygen Demand During the Pandemic” blog, vaporizers can jeopardize oxygen flow if they experience heavy icing.
However, pipe sizing inside the healthcare facility can be a less visually obvious cause of flow restriction.
“NFPA 99 doesn’t dictate pipe sizing,” says Jeff Jarvis, Medical Gas Systems Design Specialist at H.F. Lenz Company. “It just says that the piping system should be sized for adequate capacity of the load in the building.” (NFPA 99 2021 5.1.10.11.1.1) Manufacturer design guidelines and pressure drop charts are typically used to dictate pipe sizing, but they leave room for interpretation, especially in the situation of an unexpected surge in oxygen demand, such as the COVID-19 pandemic.”
“In order to determine how a new or upgraded medical oxygen pipeline system should be sized,” Jarvis adds, “it is critical to understand the potential future oxygen demands of a facility.” Oxygen demand can be estimated by reviewing the number of beds and other areas inside the hospital facility which will utilize oxygen. “Relying solely on the number of ventilators is not sufficient: vents vary. Oxygen demand for vent peak loads ranges from 10 liters/min up to 100 liters/min, complicating sizing and planning for current and future pandemics,” he notes.
Design guidelines give detailed recommendations for design of oxygen pipelines to ICUs and other high-flow areas of the healthcare facility. However, with high-flow devices such as ventilators and high-flow cannulas, flow requirements can be extremely variable, so it is important to have the support of a firm such as H.F. Lenz that specializes in medical gas piping design.
In addition to adhering to design guidelines and best practices, medical oxygen pipelines should also be sized for potential expansions, such as a new wing or tower, or the addition of hyperbaric chambers at your facility. If a medical gas pipeline system is not sized for future increases in oxygen usage, the hospital may experience undesired effects, such as pressure drops throughout the pipeline system.
Facility managers should understand the design of the existing medical oxygen pipeline to identify potential flow restrictions. Your main oxygen line should be sized to handle the current and future oxygen needs of your facility.
Existing medical oxygen pipelines should be periodically re-evaluated to determine their suitability for your future plans. Here’s why:
- Many older facilities were built before these higher-flow oxygen requirements were recognized and were often designed with the consideration that not every outlet would be in use at the same time.
- Available drawings and other documentation may not be up to date after renovations.
- Older healthcare facilities may have 3/8-inch tubing to the outlets, which was previously acceptable per NFPA. This configuration created flow restrictions at some facilities during the COVID-19 surge. Current NFPA codes state that “drops to individual station outlets and inlets shall not be less than DN15 (NPS 1/2) (5/8 in. OD) size.” (NFPA 99 2021 5.1.10.11.1.4).
Step 2: Know the new NFPA 99 Updates for 2021
NFPA 99 2021 specifies the four master alarm signals for the bulk oxygen system must also be run to the emergency oxygen supply connection (EOSC) to monitor any temporary bulk oxygen source systems that may be connected to it in the future.
- Typically, the alarms monitoring the bulk oxygen system are connected to liquid level and pressure readings originating at the bulk system (see Part 2: Understanding the Major Components of Your Bulk Medical Oxygen System) and the high/low pressure switch downstream of the bulk station located where the main line enters the healthcare facility. If you receive an alarm, call your bulk oxygen supplier. If Messer is your supplier, call Messer 24/7 at 1-800-232-4726.
- As H.F. Lenz’s Jarvis explains, “There needs to be two master alarm panels: one visible by the Responsible Facility Authority [a new NFPA-mandated position, which we’ll cover two paragraphs down] and the other at a location staffed 24/7. Each alarm panel, which also monitors other medical gases, must be hard-wired independently to maintain redundancy.” (NFPA 99 2021 5.1.9.2)
In another recent addition to the code, NFPA 99 calls for an auxiliary valve on the downstream side of the healthcare facility’s source valve (NFPA 99 2021 5.1.4.10). This creates an alternative point of connection to the EOSC if any of the bulk oxygen equipment fails or supplemental oxygen supply is required for any reason. The auxiliary valve allows for the healthcare facility’s oxygen supplier to connect a portable oxygen trailer to supply the facility from the bulk oxygen pad location. This can be a convenient solution to supply a facility that may have multiple buildings, and thus multiple EOSCs, consolidating the point of supply into one portable oxygen trailer.
In addition, NFPA 99 2021 specifies an on-site designated Responsible Facility Authority (RFA) to understand, implement and advise the hospital on code requirements. RFAs will be excellent sources of information for facility managers, respiratory therapists and others charged with supplying medical oxygen for patient care (NFPA 99 2021 5.1.14.1).*
*Please note that this is not an exhaustive list of the 2021 updates. Please refer to the text of NFPA 99 2021 for the complete list of current code requirements.
Step 3: Request a site visit
A qualified medical gas design firm can identify oxygen flow restrictions, help you better understand how you are consuming oxygen in different areas of your facility, and review your facility’s compliance to current codes and design standards. A survey of your medical oxygen pipeline can help your facility identify cost-effective solutions before small issues become larger problems during a surge.
H.F. Lenz recommends:
- Reducing oxygen consumption by closing oxygen flowmeters when not in use. Facilities should provide training to staff members to reduce oxygen waste and to prevent oxygen rich atmospheres.
- If flow restrictions are identified, installing a new, properly sized main line, parallel to the existing line. Your design firm can plan the installation around your current medical oxygen pipeline system. This work can be completed in phases to keep a hospital wing operating while the new line is commissioned.
Bulk medical oxygen suppliers and medical gas system specialists, such as Messer and H.F. Lenz, can work with your facility to identify bottlenecks in your medical oxygen pipeline system, from the bulk system all the way to the patient. Please contact Messer to schedule an assessment of your bulk oxygen system.
We deliver to you, so you can deliver to your patients.


Comments
Messer makes no warranty of any kind with respect to the subject matter, the completeness, or accuracy of this blog. Messer is not responsible for any actions (or lack thereof) taken as a result of relying on or in any way using information contained in this blog. In no event shall Messer be liable for any damages resulting from reliance on or use of information in this blog. Readers should take advice from a qualified professional when dealing with specific situations. Descriptions of, or references or access to, other publications within this blog do not imply endorsement of those publications. This blog may contain technical inaccuracies and changes to the information may be made at any time.
Gas products are hazardous. The use or misuse of gas products involves serious risks, including injury, disability and death. Users of gas products must use the Safety Data Sheets for the gas products to warn their employees and others who are exposed to the gas products or hazards associated with such products.